Prior to the Fukushima Daiichi Nuclear Power Plant accident of 2011, many people may not have heard of the word “tritium”.
Tritium (3H or T) is an isotope of hydrogen and has essentially the same chemical properties as hydrogen (H).
However, because the atom has two neutrons in its nucleus, it is an atom that is three times heavier than hydrogen.
Over a certain period of time tritium changes to a more stable isotope called helium-3 (3He).
This process is called atomic decay, during which tritium emits fast electrons (beta particles).
These are the radiation of tritium.
The energy of tritium’s beta particles is very small compared to other types of radiation, and the particles can only penetrate about 5 mm in the air.
Tritium does not escape through the walls of its container or easily pass through the skin, so external exposure to the particles is not a concern.
However, internal exposure can be a problem.
As an isotope of hydrogen, tritium exists in the environment in the form of tritiated water (HTO), which can move rapidly in the same way as regular water (H2O).
Most of the human body is water, and if that water contains tritium, the body is exposed to radiation from within.
If the tritium concentration in the body becomes high, it can be problematic for human health.
The World Health Organisation (WHO) stipulates that drinking water should have a tritium concentration of no more than 10,000 Bq/L.
Tritium is naturally generated through the nuclear reaction between neutrons contained in cosmic rays and atmospheric nitrogen.
Tritium is also a product of nuclear fission reactions, so tritium is inevitably released into the environment from nuclear facilities.
In other words, countries with nuclear power plants uniformly release tritium into the environment, which consequently becomes present in atmospheric water vapour, rainwater, seawater and tap water.
Between 1945 and the 1980s, 502 nuclear tests in the atmosphere released large amounts of tritium into the environment.
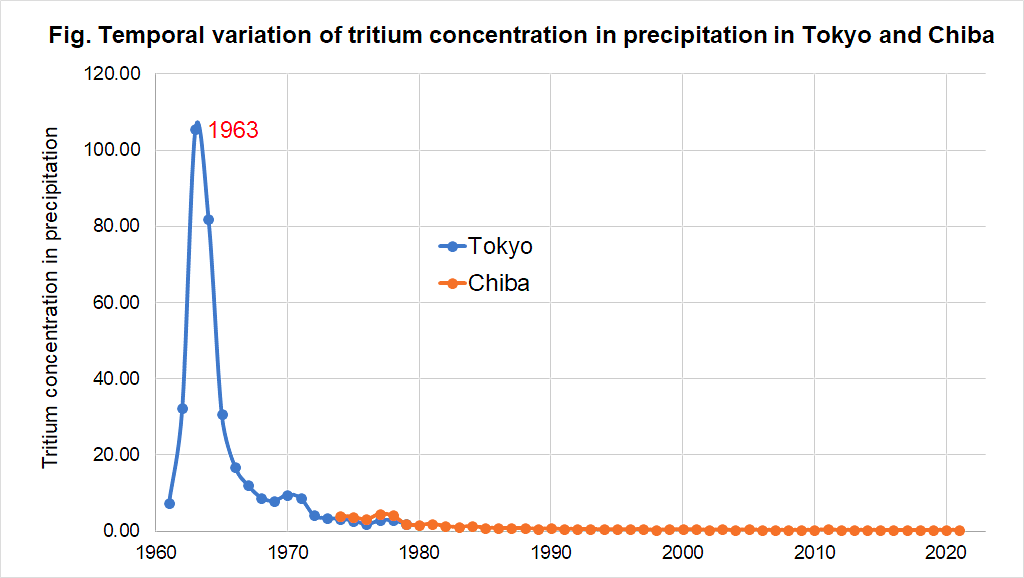
The figure shows the tritium concentrations in rainwater measured in Tokyo and Chiba.
As indicated in the figure, the tritium concentration in rainwater was very high during the 1960s when atmospheric nuclear tests were conducted, and in 1963 a value of 110 Bq/L was recorded.
After the ban on atmospheric nuclear testing in 1963, the tritium concentration in rainwater has been decreasing.
In the 1960s, it can be said that people were consuming water with a very high concentration of tritium in their daily lives.
Over time, the tritium concentration has decreased, and the current tritium concentration in rainwater is around 0.4 Bq/L.
Tritium is also present in seawater, at a concentration of approximately 0.1 Bq/L.
The table summarises the relationship between tritium concentration and exposure dose.
From this summer, ALPS (Advanced Liquid Processing System) treated water containing tritium generated following the Fukushima Daiichi Nuclear Power Plant accident will be released into the ocean.
Tokyo Electric Power Company (TEPCO) plans to release tritium into the ocean at concentrations of less than 1,500 Bq/L.
As mentioned earlier, tritium naturally exists in the environment.
The Advanced Liquid Processing System (ALPS) treated water released into the ocean is diluted by 2,000 times the same amount of seawater, making it indistinguishable from the tritium already present in seawater.
The dilution effect of seawater is significant, so the distance at which it becomes indistinguishable is only a few kilometers from the release point.
Therefore, it is extremely difficult to detect an increase in tritium concentration due to oceanic release in marine products such as seafood.
One of the concerns about the release of tritium into the ocean is that even if the concentration of tritium in seawater is low, tritium will be concentrated in vivo, and radiation exposure cannot be ignored.
Therefore, there is a theory that ocean release is dangerous. In fact, we humans live drinking water containing tritium. The tritium concentration in my body is the same as that in tap water.
Bioaccumulation of tritium is therefore not a concern.
Some people worry that tritiated water also contains other radionuclides.
Certainly, concentrations of other radionuclides are not zero.
However, the concentration is extremely low, and even if you continued to drink the tritiated water released into the ocean for a year, the value would be well below the 1 mSv annual exposure dose limit set by the International Commission on Radiological Protection (ICRP).
Note that tritiated water is diluted with seawater.
The tritiated water released into the ocean has a very high salt concentration and is not suitable for drinking as is.
However, if the salt content were removed, the water would be safe to drink.
From the standpoint of a tritium expert, there is absolutely no need to worry about TEPCO’s planned release of tritiated water into the ocean.
I would like to watch over the reconstruction of Fukushima Prefecture without unduly fearing the release of ALPS treated water into the ocean.
|
Table Tritium concentration and exposure dose |
||
|
Tritium concentration (Bq/L) |
Annual exposure dose when taking tritium continuously for one year (mSv/year)* |
|
|
WHO drinking water guidelines |
10,000 |
0.15 |
|
Tritium Concentration Limits in Canadian Drinking Water |
7,000 |
0.10 |
|
Concentration that TEPCO is considering releasing into the ocean |
1.500 |
0.02 |
|
Federal Standards for Drinking Water (USA) |
740 |
0.011 |
|
Tritium concentration in precipitation at the time of nuclear test |
110 |
0.0016 |
|
European (EU) concentration limits for tritium in drinking water |
100 |
0.0015 |
|
Tritium concentration in current precipitation |
0.5 |
0.0000074 |
|
Tritium concentration in seawater |
0.1 |
0.0000015 |
|
*Annual radiation dose is calculated based on intake of 2.25L of water (1.65L as water and 0.6L from other food) per day. |
||
Read also:
- Fallout from Fukushima radioactive wastewater
- Letter to the Editor from the Embassy of Japan on the release of ALPS-treated water
- Another scenario for Fukushima wastewater problem
- Radiation is all around us: On the safety of Japan’s release of ALPS-treated water
- Countering concerns about the release of ALPS-treated water
(Yuji Torikai is Professor at Ibaraki University, Japan.)
ADVERTISEMENT
ADVERTISEMENT