Dr Victor Hoe / Dr Nirmala Bhoo-Pathy
Before we make any rash decisions to approve any medication to treat or prevent a specific disease or symptom, there needs to be good evidence that using the medication will improve the outcome of the disease and will not cause more harm than good.
The same principle also applies when we want to repurpose a medication that has been approved for use for another disease, e.g. a broad-spectrum anti-parasitic medication for viral infection. The drug will still need to go through stringent clinical trials.
We are mindful that currently many people are feeling helpless as the COVID-19 pandemic is raging and appears to spiral out of control, and everyone is looking for a miracle.
Let us stop and learn from history: the story of thalidomide, a drug developed in 1957 originally intended as a sedative or tranquilizer.
Based on findings from early phases of its testing, the company that had developed the drug had deemed that it was harmless to humans.
With that information in hand, the drug was allowed to be sold over-the-counter without doctor's prescription.
Later on, the drug was also repurposed for treating other symptoms and diseases, e.g., colds, flu, nausea and even morning sickness in pregnant women.
While thalidomide was initially thought to be safe for pregnancy, concerns regarding birth defects arose in the 1960s.
Worldwide, more than 10,000 infants were reported to be affected and around 40% had died at the time of birth, whereas those who survived had serious limb deformities as well as eye, urinary tract or heart problems.
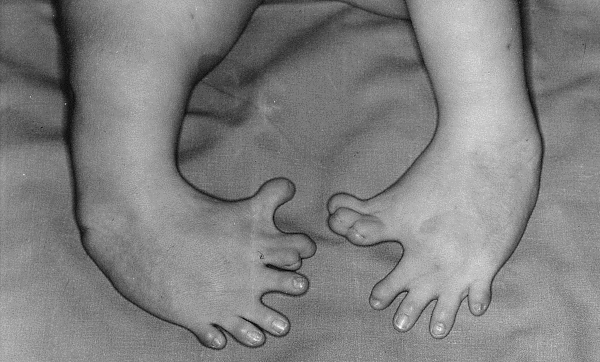
The thalidomide scandal led governments and medical authorities worldwide to ensure that any medication approved for human use should undergo stringent clinical trials and approval criteria.
The current evidence for use of Ivermectin for both the prevention as well as treatment of COVID-19 are still inconclusive.
The Cochrane Database of Systematic Review is the choice for trusted evidence for informed decisions for healthcare providers, as they have very stringent criteria for conducting systematic reviews.
A recent systematic review and meta-analysis published by Cochrane () showed a lack of evidence to support the use of Ivermectin for treating or preventing COVID-19 due to limited number of studies and small number of participants.
The systematic review found 14 studies, which included 1,678 participants that investigated Ivermectin compared to no treatment, placebo or usual care. Only one study had investigated the use of Ivermectin in preventing COVID-19.
Currently, there are another 31 ongoing studies, and 18 studies still requiring clarification from authors or not yet published that will be able to provide more scientific evidence on both the effectiveness and safety of Ivermectin.
Till then, we should wait for more concrete evidence before using Ivermectin for COVID-19, as we do not want to fall into the thalidomide trap.
Aspirin is another example of drug which was once thought to be able to improve cognitive functions based on results from early phase studies including small number of participants.
However, the findings of the ASPREE (ASPirin in Reducing Events in the Elderly) Study involving 19,114 participants found that aspirin neither reduced the risk of developing Alzheimer's disease nor delayed milder losses of memory and thinking ability as initially thought.
The bottomline is that we should not rush. We have taken the same cautious approach in the COVID-19 vaccination program for pregnant women and children.
Let science lead the way, and prevent us from falling into the thalidomide trap.
(Dr Victor Hoe is Professor of Occupational and Public Health; Dr Nirmala Bhoo-Pathy is Associate Professor of Epidemiology and Public Health, Faculty of Medicine, Universiti Malaya.)
ADVERTISEMENT
ADVERTISEMENT


































