PETALING JAYA, July 2 (Sin Chew Daily) — More than 3,000 people have signed up for the phase 3 clinical trial of COVID-19 vaccine under Yong Tai Bhd's strategic partner China-based Shenzhen Kangtai Biological Products Co Ltd.
YTB's chief executive officer and executive director Datuk Wira Boo Kuang Loon said its subsidiary YTB Healthcare Sdn Bhd received overwhelming response in its 10-day recruitment drive.
Volunteers signed up to participate in the clinical trial held at seven hospitals – University Malaya Medical Center, Life Care Diagnostic Medical Center, Columbia Asia Hospital Klang, Columbia Asia Hospital Bukit Rimau, Sri Kota Specialist Medical Center, Clinique Healthy Malaysia and ALPS Medical Center.
Boo said despite the fact more than 3,000 people have signed up for the trial, the company was still accepting names to place them on backup list.
Yong Tai advertised in major newspapers on June 21 to recruit volunteers for COVID-19 vaccine clinical trial.
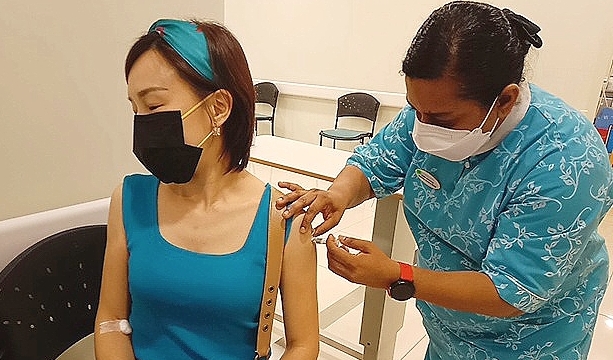
Boo said about 200 volunteers have received their first injections. As hospitals are getting more familiar with the clinical trial procedures, the vaccination rate is expected to be expedited.
All volunteers, made up of young people, factory workers and corporate sector workers, are expected to receive their first injections before July 15, he said.
None of the volunteers showed negative reactions after receiving the injection, Boo said.
He also said some of the volunteers had received their vaccination appointments while undergoing the clinical trial. They would then request for unblinding to know whether they were given vaccine or placebo.
For those who were given vaccination, they would inform the government that they did not need to be vaccinated. For those who were given placebo, they would proceed to be vaccinated.
Boo encouraged young people who have not been vaccinated to participate in the clinical trial to contribute to the development in biotechnology in the region.
ADVERTISEMENT
ADVERTISEMENT


































